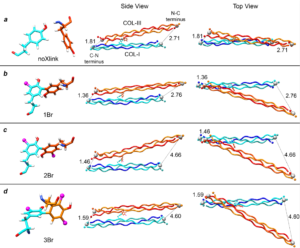
Spongin is a fundamental biopolymer that has played a crucial role in the skeletogenesis of keratosan sponges for over 800 million years. This biomaterial had so far remained chemically unidentified and believed to be an enigmatic type of halogenated collagen-keratin-based bioelastomer. Here we show collagen I and III as the main structural components of spongin. Proteomics, 13C solid state NMR and Raman spectroscopy confirm the identity of collagenous domains in spongin with collagen from mammals. Using an HPLC-MS analysis, we found halogenated di- and tri-tyrosines as crosslinking agents in spongin. Using molecular dynamics modeling, we solvated the crystal structures of collagen mimetic peptides for type I and type III collagens in four different systems, including selected brominated crosslinks. The results underscore the complex interplay between the collagen structures and crosslinks, raising intriguing questions about the molecular mechanisms underlying collagen chemistry within spongin as an ancient biocomposite.
Ehrlich, H., Miksik, I., Tsurkan, M.V. et al. Discovery of mammalian collagens I and III within ancient poriferan biopolymer spongin. Nat Commun 16, 2515 (2025).
https://www.nature.com/articles/s41467-025-57460-y

